Your basket is currently empty!
CD1d-tetramers
Case Studies for CD1d Tetramers
ProImmune CD1d tetramers provide novel insights into NKT cell activation
Barral, P. et al. (2010). CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nature Immunology. 11(4): 303-12. [PubMedID:20228797]
In the last decade, NKT cells have emerged as an essential element of host defence against microbial infection, forming an interface between adaptive and innate immunity. NKT cells respond to glycolipid antigen (such as the model antigen alpha-GalCer) presented in the context of CD1d. Crucial for the rapid pace of discovery in NKT cell biology has been the ability to manipulate and monitor NKT cells using CD1d tetramers.
Barral et al investigated the question of when and where NKT cells encounter their cognate antigen during the development of an immune response, and their comprehensive study provides exciting new insights into the dynamics of NKT cell activation.
To characterize the dynamics of NKT cells in vivo in lymph nodes (LN), the team isolated NKT cells by flow sorting cells stained with alpha-GalCer-loaded CD1d tetramers; they then fluorescently labeled the purified cells and transferred them into host mice. Imaging of intact LN showed NKT cells behaving like CD4+ T cells, moving in a random walk through their environment. To stimulate NKT cells, mice were injected with alpha-GalCer-coated silica particles. Stimulation caused a decrease in speed of labeled NKT cells within LN. The slowdown was dependent on antigen presentation, as it was not observed when CD1d-deficient mice were used to host labeled NKT cells.
Which CD1d-positive cells were presenting antigen to NKT cells in LN, and causing their retention? To narrow down the candidates, the team tracked movement of adoptively transferred NKT cells in conjunction with movement of fluorescently labeled particulate lipid. Detailed analysis of this complex experiment was performed using flow cytometry and imaging of LN. Drawing these analyses together (see figure 1) Barral et al concluded that CD169+ macrophages were mediating NKT cell arrest, forming specific and long-lasting contacts with them.
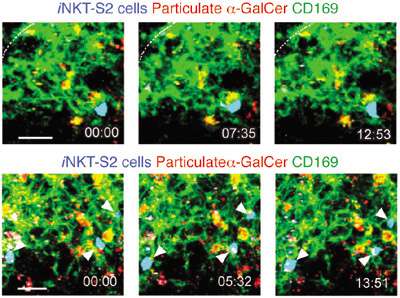
Figure 1. NKT cells arrest on SCS CD169+ macrophages. Time-lapse images from two different movies (top and bottom) showing NKT cells (arrowheads) in contact with CD169+ macrophages (green) 6 h after alpha-GalCer (red) injection. Time stamp is in mm:ss. Scale bar, 20 μm (top), 15 μm (bottom). Data are representative of six experiments.
To define the capabilities of CD169+ macrophages, LN were macrophage-depleted using clodronate liposome treatment. Injection of antigen into treated mice failed to induce full NKT cell activation and expansion. The researchers showed that CD169+ macrophages express CD1d, and are able to process an alpha-GalCer analog for presentation to NKT cells. Flow-sorted and alpha-GalCer-pulsed CD169+ macrophages were able to provoke both IFNgamma secretion and proliferation for primary NKT cells ex vivo, robustly characterizing them as capable of presenting model glycolipid antigen, via CD1d, to NKT cells.
What about antigen derived from pathogenic bacteria? Injection of mice with silica particles coated in alpha-linked galacturonic glycosphingolipid from Sphingomonas yanoikuyae gave rise to a comparable NKT cell response to particulate alpha-GalCer injection, measured by cell arrest and activation. This, again, required the presence of macrophages, as clodronate liposome treatment significantly mitigated the NKT response. Bacteria-derived NKT cell antigens thus depend on LN resident, CD169+ macrophages for their presentation.
As this study shows, the widespread availability of reliable reagents such as ProImmune’s CD1d tetramers, combined with elegant experimental design, can lead to a wealth of new information about our biology.
Figure adapted by permission from Macmillan Publishers Ltd: Nature Immunology
ProImmune’s mouse CD1d Tetramers are used to correlate NKT cell activity with protection from Type 1 diabetes.
Engkilde, K. et al. (2010). Prevention of diabetes in NOD mice by repeated exposures to a contact allergen inducing a sub-clinical dermatitis. PLoS One. 11;5(5):e10591.[PubMedID:20485668]
The Johansen Lab had previously published their observations of an inverse relationship between the incidence of Type I diabetes and allergic contact dermatitis. (Engkilde, K. et al. (2006). Diabetologia. 49(4): 644-7. PubMedID:16491393) The next logical step for Engkilde et al was to investigate a mechanism for this prevention of diabetes. When it began to emerge that NKT cells might lie at the root of the protective effects of contact dermatitis they had observed, they turned to ProImmune to supply them with CD1d tetramers for NKT cell monitoring.
Non-Obese Diabetic (NOD) mice are widely used as a model by researchers investigating type I diabetes, as the females develop diabetes from 12 weeks of age onwards. Type I diabetes is caused by Th1-type cytokine driven destruction of insulin-secreting pancreatic b-cells. Allergic conditions, by contrast, are mediated by a Th2-biased cytokine response. Since Th1 and Th2 responses are mutually inhibitory, it seems logical that provoking a Th2 reaction such as allergic contact dermatitis might protect against diabetes, but the nature of the cells making this link remain unclear.
Engkilde et al induced relatively mild allergic contact dermatitis in their NOD mice through repeated applications of p-phenylenediamidine (PPD) or 2, 4-dinitrochlorobenzene (DNCB) to the ears. As shown in figure 1, the incidence of diabetes was approximately halved in mice receiving repeated PPD applications, compared with those mice receiving DNCB, only a single dose of PPD, or water alone. The lack of an effect of DNCB may be explained by the fact that a relatively high dose was used, which could have tolerized the mice at its first application: in future, the team plan to explore the effects of lower doses.
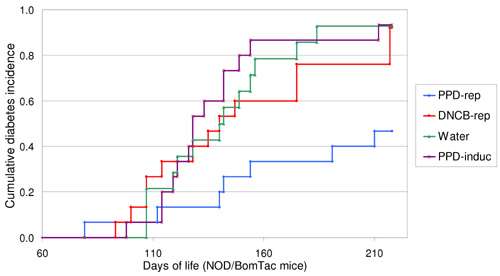
Figure 2. Repeated application of PPD inhibits the development of diabetes in NOD mice. The figure shows a Kaplan-Meier curve of the diabetes incidence for four groups of mice. DNCB-rep, PPD-rep and Water, refers to the groups of mice that were repeatedly exposed to DNCB, PPD or water every other week. PPD-induc refers to the group of mice that was only exposed to PPD in the fourth week of life. The NOD mice exposed to PPD repeatedly, displayed a cumulative diabetes incidence of 47%, in contrast to 93% of the water treated group (P=0.004). The log-rank test for all the shown curves gives a significance of P=0.008.
Histopathology performed on the ears of the mice showed a marked lymphocyte infiltration in the mice repeatedly exposed to PPD. To explore this observation further, the researchers induced contact dermatitis in cohorts of 10-week old mice through two rounds of contact allergen application. NKT cell numbers in the livers and in the auricular (ear) lymph nodes were then analyzed by flow cytometry following staining with ProImmune’s mouse CD1d tetramers. Their results were striking. As Figure 2 shows, after a second PPD application, there were significantly increased NKT cell numbers in the auricular lymph nodes.
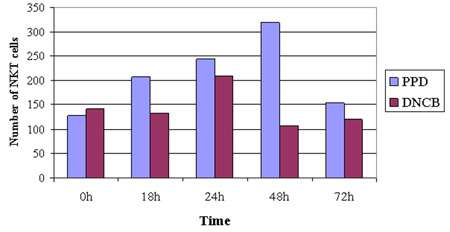
Figure 3. Topical exposure stimulates NKT cells in auricular lymph nodes. The number of NKT cells in the auricular lymph nodes (A-LN). The A-LN cells were gated to exclude nonviable cells and sample data were collected on 300,000 cells. It can be seen that both allergens stimulate NKT cells in the A-LN though PPD appears to be a stronger or longer stimulator. The increase in cells is followed by a steep drop that may be due to down regulation of the T cell receptor which has been reported previously. A Mann-Whitney U test was tests performed on the quantity of NKT in relation to the control groups (0 h) giving a significant (z=−2.095, P<0.05) result.
The data strongly suggest that NKT cells play a role in mediating protection from the onset of type 1 diabetes. Ultimately, this work could lead to the development of strategies to delay or avoid the onset of hereditary autoimmune conditions. ProImmune’s CD1d tetramers thus play a role at the forefront of valuable diabetes research.
Reproduction of figures is licensed under a Creative Commons Attribution 2.5 Generic License.
Insight gained into the significance of isoglobotrihexosylceramide (iGb3) ligand in mice using CD1d tetramer
Porubsky, S. et al. (2007). Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA. 104: 5977-5982. [PubMedID: 17372206]
The study set out to discover whether iGb3 is a physiologically relevant selecting ligand in the mouse, as it had previously been indirectly implicated as the endogenous ligand responsible for positive iNKT selection in the thymus. Porubsky et al. used ProImmune’s mouse CD1d tetramer loaded with alpha-GalCer* to determine iNKT cell frequencies in thymus, spleen and liver of mice deficient in iGb3 synthase. They concluded that iGb3 is not a physiologically relevant thymic iNKT-selecting ligand in vivo.
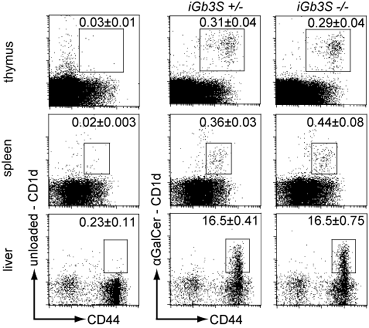
Figure 4. Thymocytes, splenocytes and hepatic leukocytes from 8- to 10-week old iGb3S+/- and iGb3S-/- mice were stained with anti-CD19 and anti–CD44 antibodies and with either alpha-GalCer-loaded CD1d tetramers to visualize iNKT population or unloaded CD1d tetramers to define the amount of non-specific binding. Plots were gated on lymphocytes. In case of spleen, CD19+ cells have been gated out. Numbers indicate the percentage of CD1d tetramer+/CD44+ cells in the lymphocyte gate + SEM; n=5. Copyright (2007) National Academy of Sciences, USA.
Case Study: Use of CD1d tetramer contributes to evidence that NKT cells are important in the acquired immune response during helminthiasis
Mallevaey, T. et al. (2007). Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infection and Immunity. 75(5): 2171-2180. [PubMedID: 17353286]
Thierry Mallevaey et al., at the Institut Pasteur in Lille, used ProImmune’s CD1d tetramer to analyze the proportions of iNKT-cell and non-iNKT-cell populations in two different strains of NKT-cell-deficient mice before and after infection with the parasite Schistosoma mansoni. The study found that the proportion of tetramer-positive (iNKT) and tetramer-negative (non-iNKT) cells among the CD3+ NK1.1+ population remained unchanged, irrespective of the time point analyzed, leading to the conclusion that both NKT-cell populations become activated 3 weeks post infection. The data suggests that iNKT and non-iNKT-cell subsets have opposite, and perhaps complementary, effects on the Th1/Th2 balance during murine schistosomiasis.
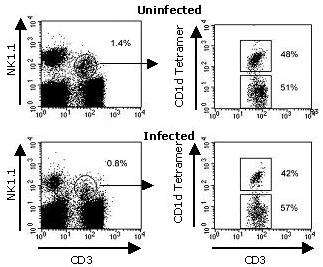
Figure 5. Analysis of splenic NKT-cell activation during S. mansoni infection. Spleens were harvested at different time points after infection and the frequency and absolute numbers of detectable CD3+ NK1.1+ cells were assessed by flow cytometry staining. Shown here are representative dot plots of total CD3+ NK1.1+ cells (0 and 7 weeks p.i., left) and tetramer+ and tetramer– cells among CD3+ NK1.1+ cells (right). Copyright (2007) American Society of Microbiology
Case study: CD1d Tetramer helps understand differentiation pathways of iNKT cells
Fedeli, M. et al. (2009). Dicer-dependent microRNA pathway controls invariant NKT cell development. J Immunology. 183(4): 2506-2512. [PubMedID: 19625646]
MicroRNAs (miRNA) are short non-coding regions of RNA that regulate gene expression by binding to complementary protein encoding mRNA. The processing of mature miRNAs is controlled in the cytoplasm by an RNase II enzyme, Dicer, which subsequently affects the development pathways of a number of cell types (e.g. B and T lymphocytes). Fedeli et al. investigated whether miRNAs were involved in the control of CD1d dependent invariant natural killer T (iNKT) cell development.
ProImmune’s mouse CD1d tetramers were used to stain cells from conditional knockout mice with one or both Dicer genes deleted. It was shown that deletion of the Dicer gene resulted in a markedly lower frequency of iNKT cells in the thymus and peripheral organs than in wild type littermates, whilst T cell lineages were unaffected by Dicer deletion. This indicates the important role of Dicer-dependent miRNA during specific iNKT cell differentiation. Further investigations revealed that this lack of detectable iNKT cells in Dicer-/- mice was due to increased cell death.
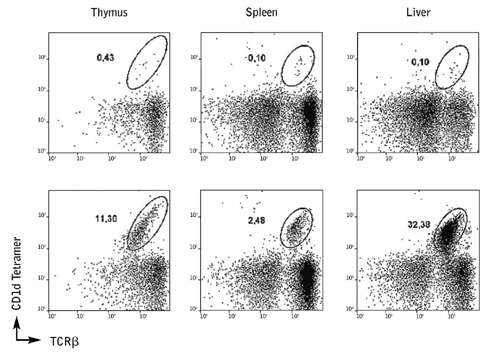
Figure 6. CD1d Tetramer staining from the thymus and peripheral organs of Dicer knockout mice show a lower number of iNKT cells compared to wild-type littermates.
Taken together these data move forward our understanding of the differentiation pathways of iNKT cells and how these differ from well described T cell lineages.
Case Study: ProImmune’s Human CD1d tetramer detects NKT cells from non-human primates
Dr. Omar Duramad from RegImmune Inc. (USA) used ProImmune’s R-PE labeled human CD1d tetramer pre-loaded with alpha-GalCer to stain PBMCs from non-human primate rhesus macaque monkeys. PBMCs from rhesus monkey were stimulated with RGI-2001 (liposomal alpha-GalCer, a ligand for NKT cells) for 3 days, followed by CD1d tetramer staining. Cells were also stained with anti-CD3 PerCP Cy 5.5 monoclonal antibodies and gated on live, CD3 positive cells.
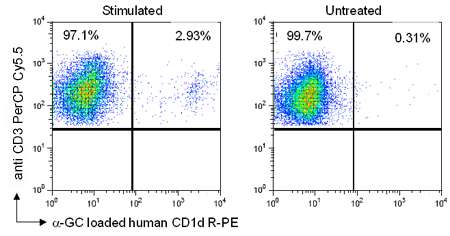
Figure 7. In PBMCs stimulated with RGI-2001, 2.93% of CD3+ cells were CD1d positive whilst in the untreated control samples only 0.31% of CD3+ cells were CD1d positive.
Thus the human CD1d tetramer from ProImmune can serve as a useful tool to enhance research using rhesus macaques.
