Your basket is currently empty!
MHC-Pentamers
Case Study:
ProVE® MHC Pentamers used for validation of novel T cell epitopes in colorectal cancer patients vaccinated with TroVax®, a novel cancer vaccine
Harrop, R. et al. (2008). Vaccination of colorectal cancer patients with TroVax® given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses.
Cancer Immunology Immunotherapy. 57(7): 977-86. [PubMedID: 18060404]
Scientists at Oxford BioMedica have used ProImmune’s PEPscreen® peptides and ProVE® MHC Pentamers to detect novel T cell epitopes in patient samples following TroVax® administration. Using ProVE® Pentamers they confirmed the MHC restriction of the T cell epitopes identified and demonstrated that the T cells were truly antigen-specific.
TroVax® consists of a recombinant vaccinia virus (MVA) encoding the tumour-associated antigen 5T4, which is rarely detected on normal tissues but is expressed at high levels on a broad range of solid tumours. The presence of the 5T4 antigen correlates with poor prognosis.
In Oxford BioMedica’s Phase I/II trial for colorectal cancer, 94% of patients responded to the TroVax® antigen. A positive correlation was found between the magnitude of the immune response and time to disease progression. Based on this information, it was of great importance for Oxford BioMedica to analyze the 5T4-specific T cell response induced by TroVax® in further detail. In particular, researchers wanted to understand the magnitude, specificity and phenotype of these immune responses.
Two subsequent Phase II studies in metastatic colorectal cancer reiterated the high frequency of responses observed in the earlier Phase I/II. In the immunological analysis of these studies, Oxford BioMedica used a ProImmune PEPscreen® 9mer peptide library in IFN-gamma ELISPOT assays to begin the validation process for 5T4 epitopes implicated in earlier MHC-peptide binding studies. This identified patients who showed no detectable 5T4-specific cellular responses prior to treatment, but who mounted very strong responses following TroVax® vaccination (Figure 1). However, the IFN-gamma ELISPOT assay indicates the frequency of cells that secrete IFN-gamma following peptide stimulation, but does not reveal their phenotype.
In order to confirm the nature of the immune responses in patients that had received TroVax®, Oxford BioMedica wished to identify the presence of increased antigen-specific T cell populations in these patients. They turned again to ProImmune for the rapid synthesis of a ProVE® MHC Pentamer Library. ProVE® Pentamers can be generated quickly and affordably for the detection of single antigen-specific T cells in flow cytometry and enable the conclusive validation of new T cell epitopes.
ProImmune supplied ten 5T4-specific ProVE® MHC Pentamers that were used to validate the responses detected by IFN-gamma ELISPOT and to confirm the MHC restriction of the 5T4 T cell epitopes (Figure 2). The precursor frequencies detected using the ProVE® MHC Pentamers were approximately 2-fold greater than those detected using the IFN-gamma ELISPOT assay against the same peptide antigen, demonstrating a good correlation between these two assays.
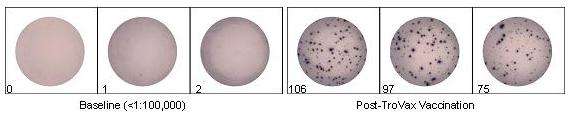
Figure 1: Patients received a total of 6 intramuscular TroVax® vaccinations: 2 before, 2 during and 2 after chemotherapy. Blood samples were taken prior to the initial vaccination and 2 weeks after completion of chemotherapy. A 96-well culture plate was coated with an anti-IFN-gamma antibody (1-D1K). 200,000 PBMCs were plated per well and incubated overnight at 37°C with 5µg/ml peptide. Cells were removed and the plate washed prior to addition of a biotinylated anti-IFN-gamma detection antibody (7-B6-1). Upon addition of streptavidin-ALP, followed by a precipitating substrate for ALP, spots developed and were counted. The figure shows results for a single patient. Prior to treatment, an average of one spot could be detected per 200,000 cells. However, following a combination of chemotherapy and TroVax® treatment, an average of 93 spots per 200,000 could be detected, indicating that a strong response to 5T4-specific peptide had been mounted.
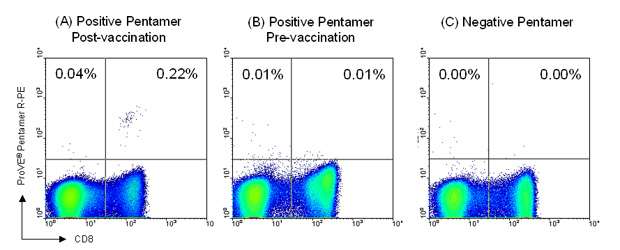
Figure 2: Patient blood samples were taken prior to the initial vaccination and 2 weeks after completion of chemotherapy. For flow cytometry staining, 2 x 106 PBMC were incubated with 1 test (0.5µg) ProVE® Pentamer for 10 minutes at room temperature, followed by 1 test R-PE-labeled Pro5® Fluorotag and 1 test FITC-labeled anti-CD8 antibody (clone RPA-T8) for 20 minutes at 4ºC. Samples were analyzed by flow cytometry and 500,000 live events collected. Results are shown for the same patient as in figure 1. A clear population of 5T4-specific CD8+ T cells was detected in the sample taken after completion of chemotherapy using an A*02:01-restricted ProVE® Pentamer (C: 0.22% of live gate). Such antigen-specific cells were not present prior to vaccination with TroVax® (B). No antigen-specific cells were detected at either time-point when an A*01:01-restricted ProVE® Pentamer (negative Pentamer) was used for staining (A).
Administration of TroVax® vaccine clearly elicits potent cellular immune responses in the patient studied, demonstrated by the expansion of antigen-specific T lymphocytes that recognize specific epitopes of 5T4. ProVE® MHC Pentamers provide a powerful means to elucidate a detailed profile of cellular immune responses in patients undergoing immunotherapy. As this study shows, CTL responses to single epitopes can be determined clearly, confirming the applicability of ProVE® MHC Pentamers in the clinical development of new immunotherapies.
PEPscreen® is manufactured by Sigma (formerly Sigma-Genosys) for ProImmune
PEPscreen is a registered trademark of Sigma-Aldrich Biotechnology LP and Sigma-Aldrich Co.
