Your basket is currently empty!
T Cell ELISPOT Assays
ProSpot® T Cell ELISpot Assays
![]()
ELISpots are best done by experienced immunologists
Outsource T cell ELISpot Assays with our convenient and reliable ELISpot service
ProImmune provides a seamless, high-throughput, core facility service for measuring cell mediated immunity in qualified T cell ELISpot assays for cyokines, such as IFN-gamma, IL-2, IL-4, IL-10, IL-13, IL-17, Granzyme B, and many others.
Save yourself the cost and effort of setting up these time-consuming ELISpot assays in your lab. Let our experienced ELISpot CRO team do the work for you, with high reproducibility and rapid turnaround. We have the capability to process tens to hundreds of samples in a project in a high throughput format, freeing up your time for core work and project planning.
By relying on our team to carry out your ELISpot assays using optimized and validated protocols, you can avoid the pitfalls of variability in cellular assays and benefit from the efficiency and accuracy of a dedicated facility.
Successful ELISpot assays critically depend on appropriate blood collection and processing – we have the experience to help you get these key areas right
We can assist you from the very early planning stages to the assessment and interpretation of results. As part of this we can either perform blood processing for you or work closely with third party cell collection and processing sites to establish cohesive protocols. Where multiple sites collect blood and isolate PBMC’s, it is essential that they use consistent techniques and process cells in a timely manner. Crucial aspects, such as cell processing, freezing and shipping procedures, can be qualified in pilot assessments prior to the initiation of the main study.
Our experience carrying out ELISpot immune monitoring ranges from phase I through multiple parallel multi-center phase III clinical trials extending over several years each. So whether your project is large or small, when working with us you will be in the right hands.
Key Publication:
Geletneky K. et al., Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa Glioblastoma trial. Molecular Therapy (2017) https://doi.org/10.1016/j.ymthe.2017.08.016
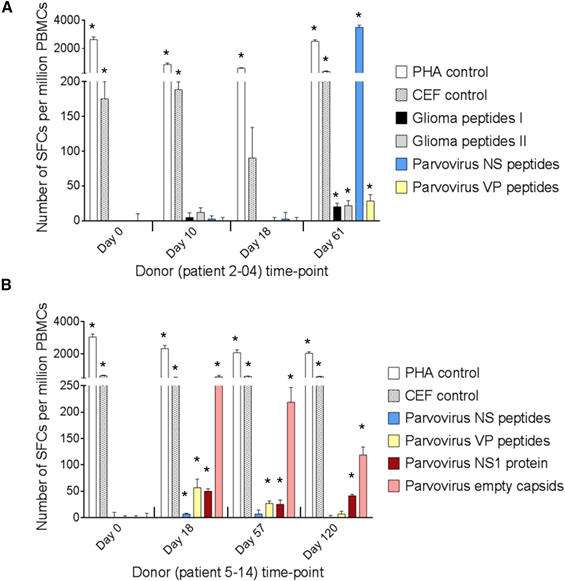
Figure 1. Data from ELISpot assays carried out by ProImmune. Evaluation of T Cell Responses to H-1PV and Glioma Antigens by IFN-γ ELISpot Assay. (A and B) Cellular immune responses are shown for two patients treated with ParvOryx via (A) the intratumoral and intracerebral route (patient 2-04) or (B) the intravenous and intracerebral route (patient 5-14). PBMCs were isolated at the indicated days prior to (day 0) or after (days 10–120) treatment. After incubation with appropriate stimulants, IFN-γ-producing spot-forming cells (SFCs) were counted. The test stimulants were viral or glioma peptides (Table S3) or full-length viral proteins (NS1 or empty capsidsmade of VP1 and VP2). Phytohemagglutinin (PHA) and cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF) peptide pools served as positive control stimulants. Negative control values (unstimulated cells) ranged from 0 to 21 SFCs per million PBMCs and were subtracted from the corresponding stimulated sample values. Means (columns) and SEMs (bars) of triplicate measurements are shown. Asterisks denote statistical significance (*p ≤ 0.05; mean SFC − 2 SEMs > 2× negative control).
When set up correctly, the Enzyme-Linked ImmunoSpot (ELISpot) assay is highly reproducible and sensitive cellular assay, particularly suited to high-throughput analysis of antigen-specific CD4+ and CD8+ T cell immune responses. It can allow detection of a secreted cytokine at the single cell level, as low as 1 cell in 100,000. For these reasons ELISpot is now a very widely used assay standard for monitoring T cell immune responses and validating new T cell epitopes.
Carrying out ELISpot assays in-house, particularly with inexperienced staff, can require significant effort and can be time consuming with variable results. You can now save time and resources, and minimize risk by relying on the expertise of ProImmune’s experienced team, who perform these assays routinely using optimized and standardized protocols with minimal variation.
Multi-center clinical trials pose the challenge of collecting, shipping and processing samples in a way that ensures consistency and repeatability. Sample logistics and preparation is probably the most important step in setting up successful assays for monitoring cell mediated immune responses. We have the experience and the capability to collect your samples wherever you are in North America and Europe and process them for maximum uniformity; sample preparation and shipping.
We have developed a streamlined service that makes outsourcing ELISpot rapid and very affordable compared to other commercial providers. Our assays are based on simple standardized formats, which follow this step-by-step process:
(1) Customer cryopreserves cells in accordance with optimized protocol recommended by ProImmune or optimized customer protocol. Alternatively ProImmune receives fresh blood in blood collection tubes and carries out cryopreservation
(2) ProImmune collects cells from customer site using our trusted shipper (customer may also use their own shipper), or, where ProImmune has processed cells, retrieves cells from ProImmune cryostores.
– ProImmune carries out HLA tissue typing as an option if required
– ProImmune carries out custom peptide synthesis as an option if required
(3) ProImmune thaws cells with optimized protocol and carries out ELISpot assays in accordance with one or more of the following assay formats:
a. ELISpot assay for detecting CD8+ and CD4+ T cell responses on frozen unmodified PBMC.
b. ELISpot assay for detecting only CD4+ T cell responses on frozen PBMC that are depleted of CD8+ T cells after thawing.
(4) Customer receives assay report and data; in addition to full analysis, the assay report includes raw data and images from the automated ELISpot reader.
On request these processes can be adapted to any additional or alternative requirements.
Unlike many other CROs, ProImmune has an unparalleled depth of experience in measuring adaptive immune responses. We also believe that time and efficiency are of the essence, which is why our service is designed to deliver unrivalled responsiveness, quality without compromise, and is more affordable than you may think.
Validation Data for IFN gamma T cell ELISpot Assay
To be confident that your ELISpot assay is consistently performing well, validation is essential. We pride ourselves on the reproducibility of our results. A sample of our validation data is shown below.
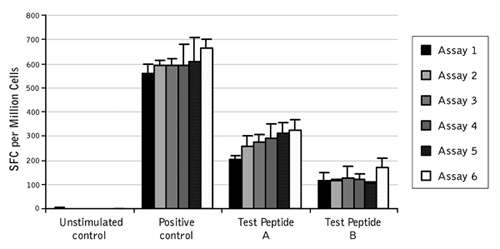
Figure 2. Low inter-assay variability in ProImmune ELISpot Assays. Normalized data from two users across six IFN gamma ELISpot assays performed over three days. The data show the consistency of the response for positive control and two test peptides.
The Statistics of ELISpot:
You may think of Shot Noise as a concept from quantum physics. In fact it is a limiting factor in cellular assays. Where such assays measure responding cells they can only do so in integer increments. For example if you are looking for a one in a million cells responding and an assay that measures e.g. 250,000 cells per condition will only have a one in four occurrence of a single response. These limitations dictate the limit of assay variance that can actually be achieved, based on the assay setup. So the question is: How close can we get to this limit?
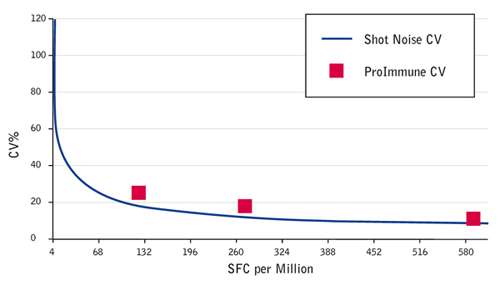
The CV achieved by ProImmune is close to the statistical CV driven by shot noise alone. Graph showing theoretical ‘shot noise’ based CV compared to the CV values achieved in ProImmune’s IFN gamma ELISpot inter assay comparison. SFC = Spot Forming Cells.
Case Study:
First ever tumor-specific response observed for human patients in oncolytic adenovirus cancer gene therapy study
Cerullo, V. et. al. (2010). Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Research. 70: 4297-4309. [PubMedID: 20484030]
Patients were treated with an oncolytic adenovirus expressing human granulocyte macrophage colony-stimulating factor (GM-CSF) driven by the viral E3 promoter to bind the expression of the transgene to adenovirus replication, and hence to the tumor environment. PBMCs from patients were isolated before and after the treatment and Interferon-gamma (IFN-gamma) ELISPOT was performed to assess whether the adenovirus treatment elicited a tumor-specific response in addition to oncolysis.
Twenty-three PBMC samples from cancer patients were supplied and ProImmune carried out IFN-gamma ELISPOT assays to determine the cytokine response to Survivin and HadV-5 ProMix™ peptide pools. The Survivin (BIRC5 PONAB) peptide pool consisted of 33 peptides, the HAdV-5 Penton peptide pool consisted of 140 peptides; the peptides in each pool were 15 amino acids long and overlapped by 11 amino acids.
Cell samples were analyzed in triplicate in the IFN-gamma ELISPOT assay to determine their potential to respond to antigen stimulation. Results from test wells were compared to those for cells that were not stimulated (no peptide/negative control), and to those for cells stimulated with phytohemagglutinin (PHA, positive control). A positive result is deemed significant if it is greater than or equal to twice the negative result (indicated by a blue dashed line on the graph).
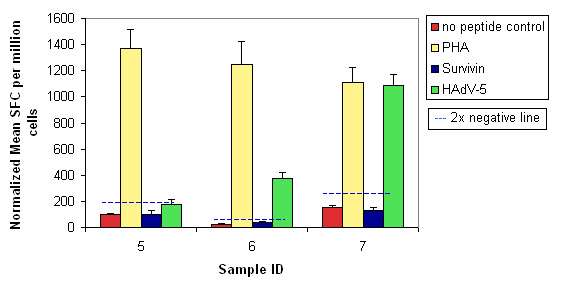
Figure 3. Sample results from one patient. Results from test wells were compared to those for cells that were not stimulated (no peptide/negative control), and to those for cells stimulated with phytohemagglutinin (PHA, positive control). A positive result is deemed significant if it is greater than or equal to twice the negative result (indicated by a blue dashed line on the graph).
The results showed that in some patients the virus treatment was able to stimulate a tumor-specific immune response to some degree. This was the first time that such a response had been observed in human patients treated with an oncolytic adenovirus.
