Your basket is currently empty!
typeHLA: HLA Typing Service
typeHLA: HLA typing simplified
the world’s leading HLA typing service for HLA analysis in clinical cohorts

Over 99% of our genes are the same – HLA type is a crucial part of the difference
HLA typing now costs less: get your quote today
HLA Tissue Typing Service
|
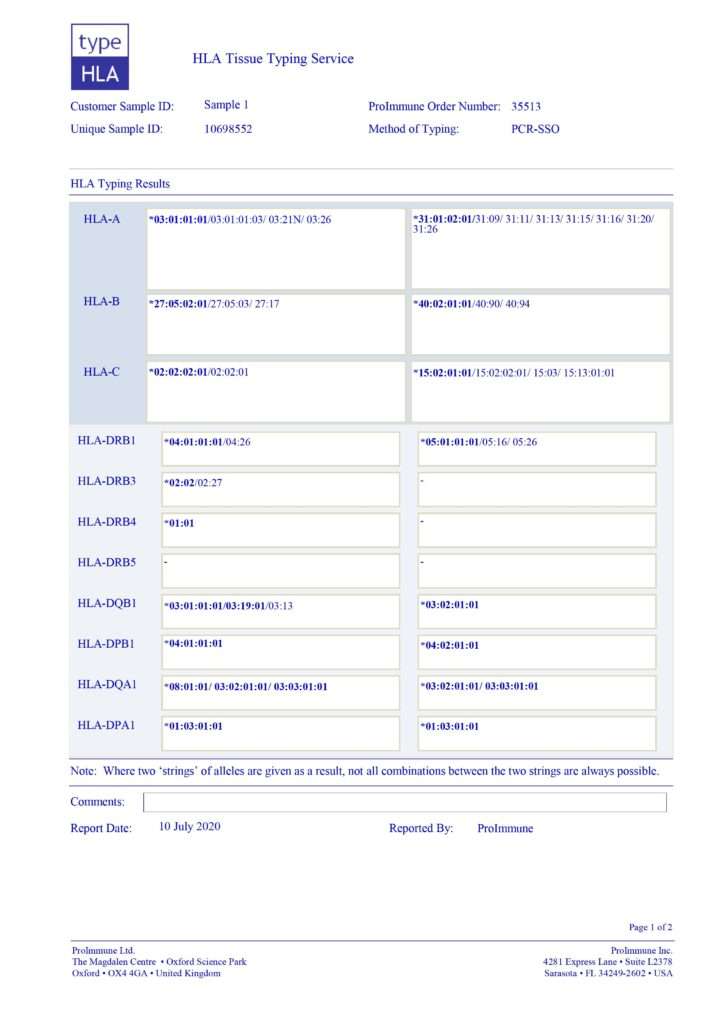
Figure 1. typeHLA sample report: clearly presented, easy to interpret
| HLA typing your study cohort – it could make all the difference | ||
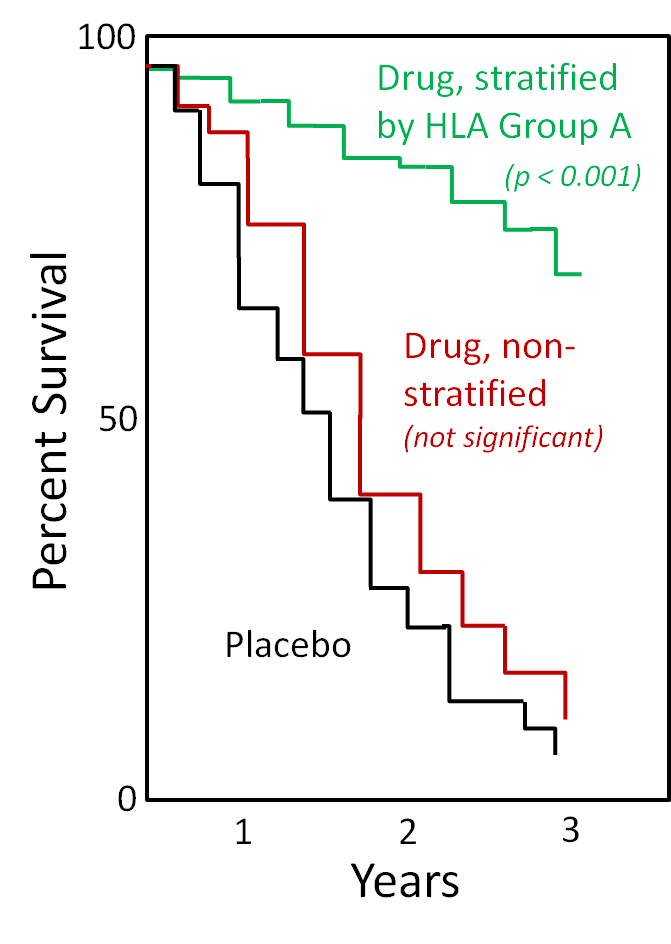 Figure 2: impact of trial stratification by HLA type (for illustration purposes only; actual results may show different trends) Figure 2: impact of trial stratification by HLA type (for illustration purposes only; actual results may show different trends) |
HLA typing is inexpensive compared to the cost of running most clinical studies and an excellent risk management tool that can help protect your investment in time, effort and money.
Many clinical studies fail their primary endpoints because they do not reach statistical significance on their core hypothesis. Studies usually cover individuals with a broad distribution of HLA types. However, when a study is stratified based on detailed HLA typing data, previously unseen statistically significant trends can become visible. This can change failure of a study overall into success in a sub-group, helping you to protect your investment. Equally adverse events, such as drug immunogenicity, may be HLA linked. Where such events become a problem for your study overall, it’s good to know HLA associations where they do exist, giving you better choices for progressing your work. |
|
typeHLA Tissue Typing Service Overview
| Typing is available locus by locus as follows: | ||
| Class I loci available | HLA-A, B, C (can be ordered individually) | |
| Class II loci available | HLA-DRB1, DRB3/4/5, DPA1/DPB1, DQA1/DQB1 (can be ordered individually) | |
| Typing resolution | 4 digit (allelic level) typing but with some degeneracy | |
| Turnaround time | Approximately 2-3 weeks (depending on sample number) | |
| Sample formats | gDNA or (with extra charge): cryopreserved PBMCs and other cells, blood, saliva | |
| Report format | Electronic format (PDF, XLS) via secure portal | |
Published example for HLA linked adverse outcome of IFN beta therapy in multiple sclerosis
Link J et al.* (2014) “Human Leukocyte Antigen Genes and Interferon Beta Preparations Influence Risk of Developing Neutralizing Anti-Drug Antibodies in Multiple Sclerosis”
*Department of Clinical Neuroscience / Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden
PLoS ONE 9(3): e90479. doi:10.1371/journal.pone.0090479
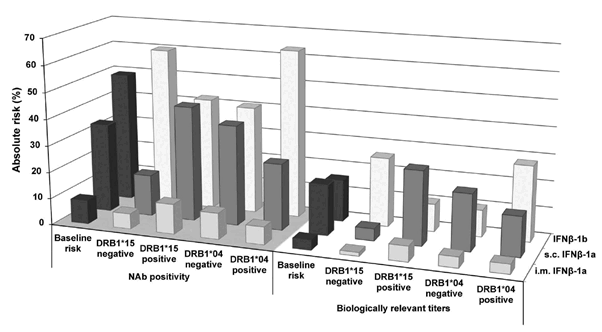
Figure 3. Absolute risk for development of neutralizing anti-drug antibodies (NAbs) and biologically relevant titers.
Calculation of the absolute risk was used to estimate how DRB1*04 and DRB1*15 carriage impacts the risk for the outcomes of NAb positivity (bars on grey floor) and biologically relevant titers (bars on white floor) when adjusted for the baseline risk. Baseline risk was the frequency of development of NAb (dark grey bars, grey floor) and biologically relevant titers (dark grey bars, white floor) for each IFNb preparation in the Swedish NAb registry. Compared to the baseline risks, DRB1*15 carriage increased the absolute risk for both outcomes in s.c. IFNb-1a treated patients (grey bars) and to a lesser extent in patients receiving i.m. IFNb-1a (light grey bars), whereas DRB1*15 carriage lowered the risk for both outcomes in patients receiving IFNb-1b (white bars). In DRB1*04 carriers the reversed relationship was observed, with an increased absolute risk for both outcomes in IFNb-1b treated patients. Moreover, the absolute risk for NAb positivity and biologically relevant titers was constantly lower for i.m. IFNb-1a than for the two s.c. IFNb preparations, regardless of whether patients were positive or negative for DRB1*04 and DRB1*15.
typeHLA Tissue Typing Service in Detail
Using PCR-SSO the genomic DNA is amplified using PCR, then incubated with a panel of different oligonucleotide probes, which have distinctive reactivities with different HLA-types. Typing resolves major allele groups to 4 digits, with some degeneracy in allele resolution, e.g. HLA-A*23:01/03/05/06.
Sample types and order handling
We accept genomic DNA, saliva samples, fresh or frozen whole blood or frozen cells, and will provide you with details of how to ship each of these to us. There is a handling charge for samples that are not submitted as genomic DNA.
We offer a worldwide service, available for one sample or hundreds – our high throughput service enables us to process tens to hundreds of samples at a time. You can confidently send your samples from any location worldwide using our experience in shipping globally.
Sample requirements and sample quality
We have the following requirements for samples:
- gDNA: Minimum 15 ng/μl concentration and minimum 100 μl. Quality must be sufficient to allow production of amplicons of 5.5.
- Blood: Fresh or frozen, 4 ml drawn into EDTA.
- Cells – cryopreserved or snap-frozen PBMCs or other nucleated cells: 1 vial of 1-2 million cells.
- Saliva: collected in Oragene-ONE (Worldwide) or Oragene-DISCOVER (USA) kit.
Note that samples provided as whole blood/PBMC/saliva are subject to an Order Handling Fee. Samples supplied as gDNA are not subject to the Order Handling Fee.
Key publication:
Nyanhete T. E.. et al., “HLA class II-Restricted CD8+ T cells in HIV-1 Virus Controllers” Scientific Reports (2019),Vol. 9, Art.No. 10165
https://www.nature.com/articles/s41598-019-46462-8
Abbreviated summary:
A recent study highlighted the presence of HIV-1-specific class II-restricted CD8+ T cells in HIV-1 patients who naturally control infection (virus controllers; VCs). We show that memory class II-restricted CD8+ T cell responses are more often detectable in VCs than in chronically infected patients, but not in healthy seronegative donors. We also demonstrate that VC CD8+ T cells inhibit virus replication in both a class I- and class II-dependent manner. These data demonstrate that anti-viral memory class II-restricted CD8+ T cells with hybrid CD4+ and CD8+ features are present during natural HIV-1 infection.
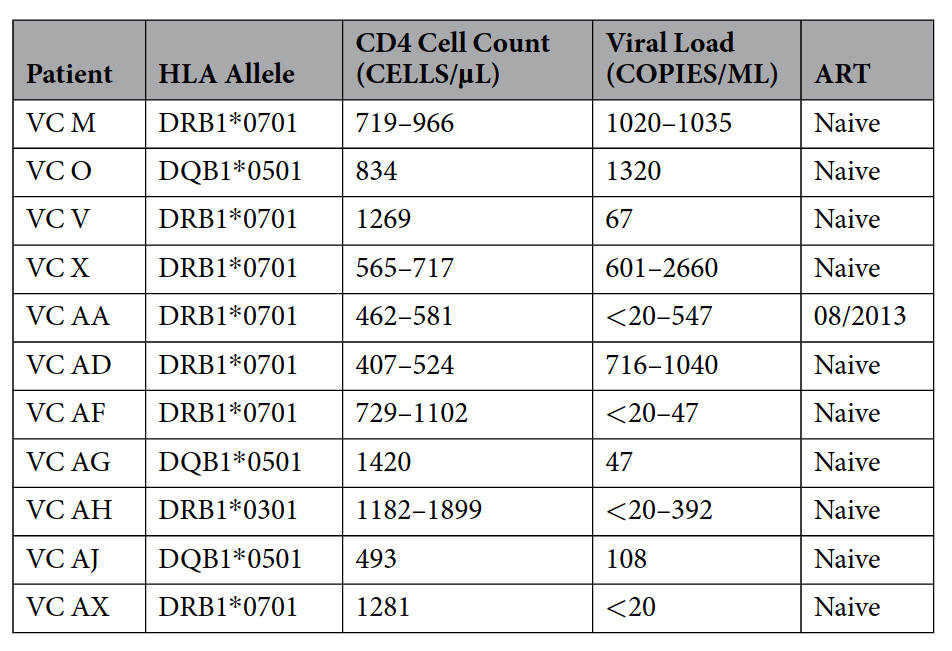
Table. HIV-1 Virus Controller Cohort. List of HIV-1 VC patients used in the study. VC AA went onto ART after enrollment into the study, and hence time points before and after ART enrollment were used when determining the frequency and memory phenotype of class II-restricted CD8+ T cells. HLA typing performed by typeHLA.
Why use our typeHLA service to take care of your HLA typing requirements?
- Uses state of the art genotyping technologies developed for HLA typing in clinical applications;
- Our service covers study planning, sample shipping, data reporting and interpretation as well as an appropriate contractual relationship;
- Eliminates issues that can arise from informal relationships with a local typing lab;
- Professional, reliable HLA Tissue Typing service;
- In advance of starting functional cellular assays such as ELISpot, or carrying out flow cytometry testing with HLA multimers, tissue typing is usually required for interpreting results successfully;
- If you are carrying out a clinical trial, establishing the tissue type of your donor samples is an important step. Many diseases and the immunological response to them are associated with specific HLA types. Correlating your trial results with the sample tissue type may give you critical additional information that could make a difference to the interpretation of the results;
- FDA Guidance on Immunogenicity Assessment for Therapeutic Protein Products (August 2014) recommends as follows: “Evaluation of genetic factors that may modulate the immune response to a therapeutic protein product is recommended. For example, the subset of patients that generate neutralizing antibodies to IFN-beta products are more likely to possess distinct HLA haplotypes (Hoffmann, et al. (2008) Am J Hum Genet, 83(2):219-27). Thus knowledge of the heightened susceptibility of patients with such HLA haplotypes may allow for measures to prevent such responses or to pursue other treatment options.”
- If your time and resources are limited, and deadlines are imminent, our typeHLA Service provides just the help you need.
Praise for typeHLA from:
Dr. Jennifer Kirchherr at the Duke Human Vaccine Institute in North Carolina, USA
“We looked at a range of companies offering tissue typing, and also at using an on-campus typing facility, and we found that ProImmune were able to offer us the best pricing and turnaround time.”
Dr. Navapon Techakriengkrai at Chulalongkorn University, Thailand
“I outsource my tissue typing to ProImmune because they provide me with the fastest service at the best price. Since the main theme of my research is on T cell immunology, HLA-type is inevitably needed. The customer service from ProImmune is of the best quality.”
More on HLA-linked diseases and treatments
An individual’s immune response to a foreign agent, such as a vaccine, drug or allergen, can be strongly influenced by their HLA tissue type. In addition there are numerous examples of diseases with known HLA association – for instance, Type 1 Diabetes is associated with HLA-DRB1*04:01, Multiple Sclerosis with HLA-DRB1*15:01 and protection from HIV infection with B*57:01 and B*35:01. It seems absurd, but HLA type is ignored in most clinical trials where the presence of a novel therapeutic is known to influence the immune response.
One of the most relevant examples is recombinant factor VIII, used to treat suffers of hemophilia. There is clear bias towards an anti-drug response in a subset of recipients with common HLA alleles. Efforts are underway to understand this bias fully, and to develop variants of Factor VIII that will be less immunogenic for these individuals.
Autoimmune diseases including Rheumatoid Arthritis (RA) show a strong association with HLA DRB1*01:01 and HLA-DRB1*04:01. A therapeutic target in RA is CD20, and the anti-CD20 antibody Rituxan® (Rituximab), (a chimeric mouse/human monoclonal antibody developed by IDEC Pharmaceuticals) is used in RA treatment. However, Rituxan® contains DR1 and DR4 restricted T cell epitopes, which in the RA population contribute to a high rate of reported immunogenicity.
These examples illustrate the case for understanding your patient population in terms of HLA type, and thus knowing at an early stage how your biologic may perform in different populations. Even if your clinical trial doesn’t result in your therapy going forward, you can maximize the amount of information you gain from the trial by having HLA typing data available.
