Your basket is currently empty!
ProPresent® MAPPS Antigen Presentation Assay | ProImmune
ProPresent® MAPPS Antigen Presentation Assay
discover more with the world’s leading MHC-associated
peptide proteomics (MAPPS) assay
ProPresent® is ProImmune’s MHC-associated peptide proteomics (MAPPS) assay. It lets you directly identify the peptides which are presented by antigen-presenting cells to T cells. Our core assay has a turnaround time of just 3-4 weeks.
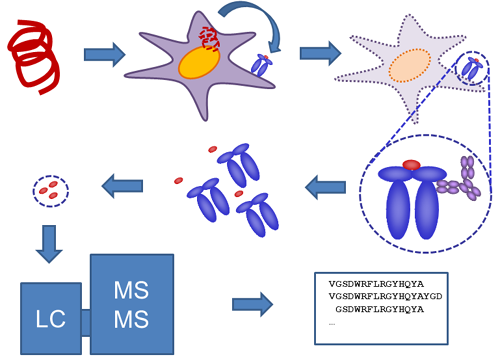
Figure 1: ProPresent® example workflow when measuring presentation from protein or protein mixture. Antigen-presenting cells take up the protein and process/present peptide fragments bound in MHC molecules on the cell surface. Cells are lyzed and HLA-peptide molecules recovered in an immune affinity step. Peptides are recovered from the HLA molecules and analyzed by LC-MS/MS. Identified sequences are subjected to rigorous analysis to identify true positive peptides with high confidence.
ProPresent® tells you exactly which epitopes from your biotherapeutic drug or other protein of interest are presented by HLA molecules to T cells. Peptides are identified by the classical method of HLA-peptide complex extraction, peptide elution and subsequent peptide epitope identification by sequencing mass spectrometry.
ProPresent® can be used to identify the peptides associated with HLA-DR, DP or DQ, and with HLA Class I. Combined with ProImmune’s REVEAL® HLA-peptide binding assays and functional T cell assays, ProPresent completes the picture in understanding the potential immunogenicity of your compounds.
Watch the in-depth FDA study using the ProPresent® MAPPS assay presented by Dr. Zuben Sauna at our Mastering Immunity conference
Key publication by team at FDA investigating immunogenicity of Cas9 protein
Simhadri V.L., Sauna Z.E., et al., Nature Communications (2021) “Cas9-derived peptides presented by MHC Class II that elicit proliferation of CD4+ T-cells”
(read the full publication here)
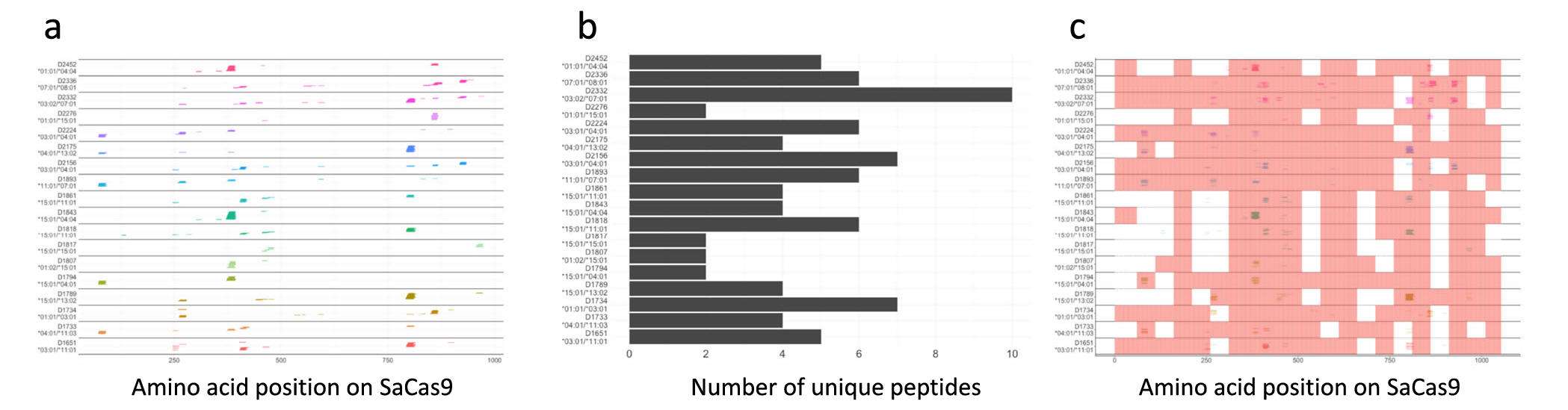
Figure 2: Peptides identified in the ProPresent® assay. a. Peptides are shown with their positions on the SaCas9 protein (depicted on the X-axis). The peptides identified are shown individually for each of 18 donors. The HLA-DRB1 alleles associated with the donors are depicted on the Y-axis. The peptides are stacked to show multiple peptides detected at each position on the Cas9 sequence for each donor. b. The number of unique, continuous SaCas9 peptides detected on DCs from each donor. c. Results of the ProPresent Assay (colored lines) are overlayed on the results of the flow cytometry-based T-cell proliferation assay (pink areas). We assumed that donors were a match if they shared at least one HLA allele.
Short summary: The objective of this study was to map immunogenic T cell epitopes from Staphylococcus Aureus Cas9 enzyme (SaCas9) epitope mapping was carried out for the full length SaCas9 sequence based on several cell-based assays including ProPresent® to directly measure peptides that are processed and presented by multiple donors. Overall, 22 SaCas9 peptides were identified that are both presented by MHC-II proteins and stimulate CD4+ T cells. While there are no adverse events related to Cas-protein immunogenicity reported up to date, the clinical assessment of safety and efficacy of CRISPR-Cas-mediated gene therapy would benefit from the improved immunogenicity risk assessments using the tools reported in this study. As more clinical data emerges on immune responses to CRISPR-Cas proteins, these tools can be used to better profile and understand the impact of immunogenicity on clinical safety and efficacy.
Key publication by team at CSL Behring investigating reduced immunogenicity of sc-rFVIII AFSTYLA®
M. Hofmann, et al. (2017) “Analysis of the Novel Recombinant Factor VIII-SingleChain Protein Predicts A Lower Immunogenic Potential as Compared to Full-length Recombinant FVII”
(read the full abstract here)
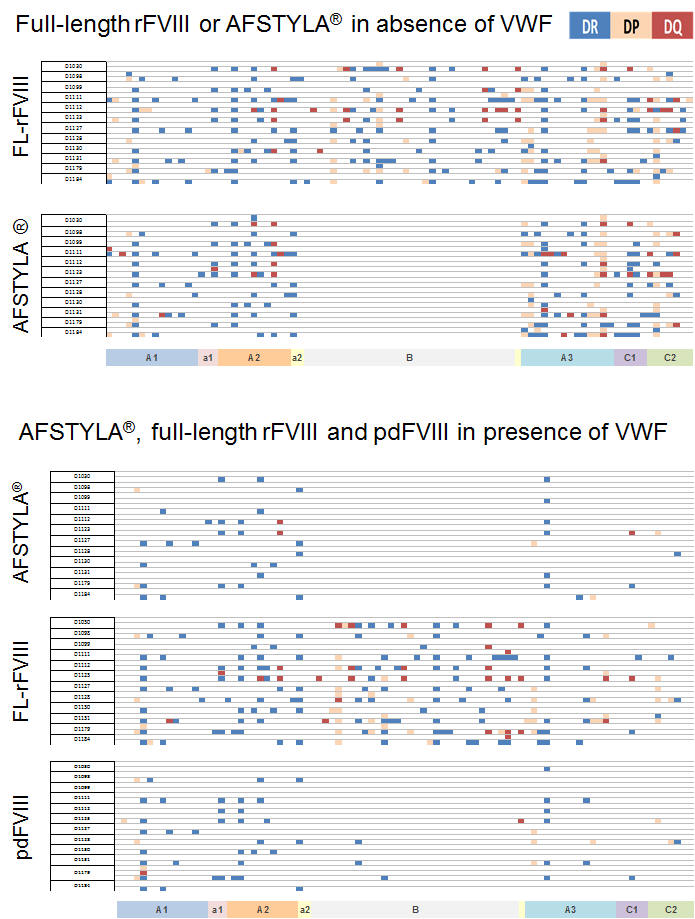
Figure 3: ProPresent® assay to investigate modulation of antigen presentation of FVIII by VWF. FL-rFVIII expressed more unique epitopes than AFSTYLA® in the absence of pdVWF due to B domain truncation. Complexing with VWF (in a physiologic relevant ratio) dramatically reduced the generation of T cell epitopes for AFSTYLA® to that observed with pdFVIII in presence of VWF. In contrast, T cell epitopes generated from FL-rFVIII were poorly inhibited by VWF *. Five (magenta boxes, top lower panel) out of 43 uniquely or more frequently presented FL-rFVIII-derived epitopes (identified in presence / absence of VWF) triggered T cell proliferation in a ProMap® study**. A previous ProPresent®/ ProMap® identified 27 FL-rFVIII-B domain derived and one T cell epitope common for FL-rFVIII and BDD-rFVIII, not present in AFSTYLA® (data not shown).
*Healthy donors (n=12) were selected as source of moDCs with HLA-DR/DP/DQ covering ~80% of global population. One row of each data set shows HLA-DR/DP/DQ presented T Cell epitopes from donor 1-12 plotted against FVIII sequence with allele specific color-code
** Healthy donors (n=40), <98% population coverage, n=57 synthetic overlapping peptides tested
Short summary: AFSTYLA® developed by CSL Behring is the first and only single chain recombinant FVIII molecule designed to increase dosing intervals while providing high clinical efficacy. CSL Behring used ProImmune’s ProPresent® antigen presentation assay to investigate AFSTYLA® for antigen presentation by dendritic cells via HLA DR, DQ and DP. It was found that antigen presentation for AFSTYLA® is reduced compared to other commercial rFVIII products. Follow-on ProImmune ProMap® T cell assays also showed a reduced T cell epitope response profile for AFSTYLA®. Overall the findings from such pre-clinical assays are consistent with clinical results for AFSTYLA® that are already available, where no inhibitors to AFSTYLA® were found (read the full abstract here)
ProPresent® example applications
- Determine protein antigenicity
- Identify which antigens from an oncogene, virus, bacteria, pathogen, vaccine or other vector are actually presented on cells
- Discovery of new biomarkers
- Discover new peptide targets for cancer and infectious disease interventions
- Understand the impact of protein modifications on immune responses
- Separate analysis for HLA-DR, DP, DQ, pan HLA-ABC, HLA-A2, mouse H-2 available
|
|
|
|
|
|
ProPresent® and Immunogenicity
Knowledge gained from ProPresent® and ProImmune’s REVEAL® Immunogenicity services provides you with the information needed to understand and manage immunogenicity of biotherapeutics or other protein compounds. Our system permits comparison of data from a set of donor samples and can help explain whether patients with particular HLA-types could be at higher or lower risk of an adverse reaction to a biological compound. The assay is compatible with fully formulated biologics and can also be used to compare different proteins, or different formulations of the same protein for an alteration in the pattern of presented epitopes. ProImmune’s whole protein DC-T cell and peptide T cell proliferation assays can be employed to confirm functional relevance of the epitopes identified by ProPresent®.
Applications for the ProPresent® Antigen Presentation Assay
-
Epitope discovery and characterization
- Epitope discovery and characterization
- Establish epitopes for monitoring a patient response to a vaccine or biologic
- Profile known or suspected allergens
- Identify the immunological impact of sequence variants of the same protein, e.g. in viral proteins, or for tumorigenic point mutations
- Investigate the population bias of epitope responses using HLA-typed donors
-
Risk Assessment of Biologics
- Identify presented epitopes in food additives or other consumer goods, e.g. cosmetics
- Identify presented epitopes in pharmaceuticals and other biological products
- Compare and contrast the presented epitopes from different batches, formulations, or production methods for the same biologic, to pre-empt safety concerns
-
Profile the responses to biosimilars and biobetters
-
Establish a baseline for safety assessment; compare novel agents to established, safe, comparator proteins
-
Generate data to support a regulatory submission
Detailed Process Flow for ProPresent® Antigen Presentation Assay Service where ProImmune Provides Dendritic Cells
- Protein is supplied by the customer
- A panel of HLA-typed, healthy donor peripheral blood mononuclear cell (PBMC) samples are prepared from the ProImmune tissue bank (selected to reflect HLA distribution of choice)
- Monocytes from donor PBMC are cultured in defined media and differentiated to produce dendritic cells (DC)
- DC are loaded with the test antigen and induced to mature
- DC are harvested, HLA molecules are purified and the associated peptides are eluted
- Peptide samples are analyzed by sequencing mass spectrometry
- The mass spectrometry data is compared against a protein database library consisting of the sequence of interest and the Swiss-Prot database of the organism of choice
- Peptides are ranked by significance according to a probability based algorithm
- Data are verified by searching against a scrambled decoy database to reduce false positives
- A full data report is compiled, listing all detected epitopes including presentation of nested epitope sets and anchor analysis against characterized HLA alleles with a summary of detected control proteins.
- Delivery time can be approximately 6 weeks, depending on the scope of the project
Frequently-Asked Questions
Can ProPresent® be used to investigate antigen presentation by cryopreserved cells?
Yes. We can extract the MHC-peptide complexes from well-preserved cells to use in ProPresent® and give you a unique insight into the peptides present on their cell surface.
How do I validate the epitopes identified by ProPresent®?
We recommend testing the peptides in our Naïve T cell CFSE proliferation assays, but there are a range of options available – simply ask us which is best for you.
ProPresent® in Context
ProImmune provides several modular tools for understanding immune responses. The in vitro methods we offer can define sequences within an antigen that can bind to HLA molecules, and whether or not these sequences cause T cell responses. However, functional assays do not reflect the many complex internal cellular processes important in the presentation of antigens by HLA Class II. These processes are accounted for using the ProPresent antigen presentation assay, which determines the repertoire of naturally presented peptides in antigen-pulsed DC. The methodology automatically includes natural editing activities, such as protease-based cleavage and peptide editing by HLA-DM and other antigen presentation pathway components.
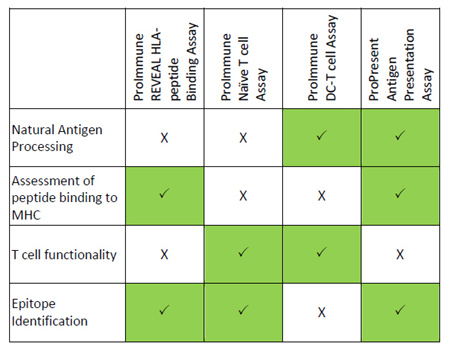
The following table summarizes key elements that form part of an ideal immunogenicity risk assessment strategy for a biological compound and which of ProImmune’s services is most appropriate for each stage.
Key publications for ProPresent®:
ProPresent used by Bavarian Nordic to identify cancer vaccine (TVH)-derived antigens
Preclinical development of a first-in-class vaccine encoding HER2, Brachyury and CD40L for antibody enhanced tumor eradication Hinterberger (2023) Scientific Reports 13:5162
ProPresent used by Takeda to study tolerogenic liposome treatment of neuromyelitis optica
The evaluation of lymph node cell proliferation response by liposomes loaded with major histocompatibility complex class II binding aquaporin 4 antigen peptide Muraki (2021) Bioscience, Biotechnology and Biochemistry 85:3, 537–544
Team at FDA and Editas identify naturally presented and functionally relevant T cell epitopes from CRISPR-Cas9
Cas9-derived peptides presented by MHC Class II that elicit proliferation of CD4+ T-cells Simhadri (2021) Nature Communications 12: 5090
Team at FDA and CSL Behring investigate the presentation of FVIII-derived peptides in monocyte-derived DCs from hemophilia patients as well as healthy donors using ProPresent
Peptides identified on monocyte-derived dendritic cells: a marker for clinical immunogenicity to FVIII products Jankowski et al (2019) Blood Advances 3:1429
Team at Novo Nordisk and FDA investigate the immunogenicity of engineered F-VIIa
Post hoc assessment of the immunogenicity of bioengineered factor VIIa demonstrates the use of preclinical tools Lamberth et al (2017) Science Transl. Med. 9:372
Team at Nutricia Research and Utrecht University investigate the tolerogenic potential of hydrolyzed milk formula
Identification of Peptides with Tolerogenic Potential in a Hydrolyzed Whey-Based Infant Formula Gouw et al (2018) Clin Exp Allergy 48: 1345
AAPS Meeting report published by authors from Amgen, Novartis, Genentech and ProImmune:
Immunogenicity of Antibody Drug Conjugates: Bioanalytical Methods and Monitoring Strategy for a Novel Therapeutic Modality Hock et al (2015) AAPS Journal 17: 35
Team at Pfizer explores antigen presentation for therapeutic monoclonal antibodies
Contribution of enhanced engagement of antigen presentation machinery to the clinical immunogenicity of a human IL-21 receptor‐blocking therapeutic antibody. Xue et al (2016) Clin Exp Immunol 183: 102
Team at Sanofi Pasteur confirms CMV epitope with ProPresent® Read case study
HLA-DR and HLA-DP Restricted Epitopes from Human Cytomegalovirus Glycoprotein B Recognized by CD4+ T-Cell Clones from Chronically Infected Individuals Ventura et al (2012) J Clin Immunol 32: 1305