Your basket is currently empty!
ProVE® Pentamer Libraries
ProVE® MHC Class I Pentamer Libraries
for cost-effective, definitive validation of new CD8+ T cell epitopes
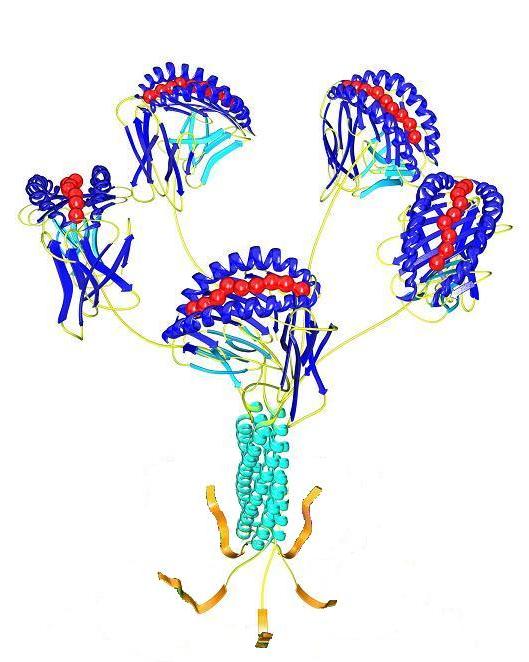

Figure 1: ProVE® Pentamers generated by rapid, high-throughput, parallel synthesis. They comprise five MHC-peptide complexes assembled through a coiled coil domain. Due to their planar configuration, all five MHC-peptide complexes in the Pentamer are available for binding to complementary T cell receptors. A Pro5® Fluorotag or Biotag is supplied (R-PE, APC or biotin) for flexible two-layer staining.
Pentamer guided epitope mapping with ProVE® Pentamer Libraries
ProVE® MHC Class I Pentamer Libraries provide a quick and cost effective way of screening a number of peptides implicated as epitopes in the immune response under investigation.
ProVE® Libraries bridge the gap between the screening of high numbers of T cell epitopes and the in-depth monitoring and characterization of single specificity CD8+ T cell immune responses with our individually manufactured Pro5® MHC Class I Pentamers. A cost reduction of more than 80% is achievable compared to the synthesis of standard MHC reagents.
ProVE® Pentamer Library Features |
|
| Rapid high throughput synthesis process | Fast delivery time (2-3 weeks). A large number of Pentamers can be delivered together. |
| Flexibility in supplying the peptide | Custom peptides can be synthesized by ProImmune or supplied by the customer. |
| Full QC for each Pentamer | Consistent and reliable performance compared with self-made tetramers or self-loaded dimeric MHC products. |
| Low price per multimer and no custom set up charges | Up to 80% cost reduction per reagent when compared to the synthesis of standard Pentamers |
Example Published ProVE® Pentamer Staining Data:
Harrop, R. et al. (2008). “Vaccination of colorectal cancer patients with TroVax® given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses.”
Cancer Immunology Immunotherapy. 57(7): 977-86. [PubMedID: 18060404]
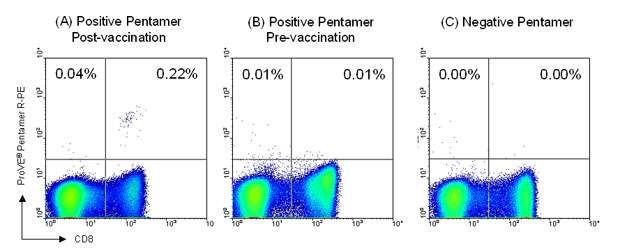
Figure 2: Patient blood samples were taken prior to the initial TroVax® vaccination and 2 weeks after completion of chemotherapy. For flow cytometry staining, 2 x 106 PBMC were incubated with 1 test (0.5µg) ProVE® Pentamer for 10 minutes at room temperature, followed by 1 test R-PE-labeled Pro5® Fluorotag and 1 test FITC-labeled anti-CD8 antibody (clone RPA-T8) for 20 minutes at 4ºC. A clear population of 5T4-specific CD8+ T cells was detected in the sample taken after completion of chemotherapy using an A*02:01-restricted ProVE® Pentamer (C: 0.22% of live gate). Such antigen-specific cells were not present prior to vaccination with TroVax® (B). No antigen-specific cells were detected at either time-point when an A*01:01-restricted ProVE® Pentamer (negative Pentamer) was used for staining (A). Read the full case study here
Product Specification
A ProVE® Pentamer Library is a set of custom Pentamers. The customer specifies the MHC allele under investigation and provides a small quantity of each of the peptides of interest. Alternatively, a custom synthesis of the chosen peptides may be ordered from ProImmune at competitive rates.
ProImmune manufactures the Pentamers using a rapid, high throughput, parallel synthesis process and supplies them unlabeled and at a minimum quantity of 20 tests. There is a minimum order of 10 Pentamers per ProVE® Library per allele.
ProVE® Pentamers are suitable for multiplexed staining of antigen-specific T cells in flow cytometry. This enables the user to identify and quantitate different populations of single antigen-specific CD8+ T cells very rapidly and attain conclusive validation of new T cell epitopes.
Quality Control
Each ProVE® Pentamer is QC tested. The protein concentration is determined and translated into quantity for each product. The customer pays for the complete synthesis run and receives those ProVE® Pentamers that pass quality control. The average QC pass rate for HLA-A*02:01 ProVE® Pentamers is 85% for peptides with scores >21 in the SYFPEITHI epitope prediction algorithm (www.syfpeithi.com).
Before proceeding with an order, ProImmune will review the sequences of the peptides submitted for inclusion in the ProVE® Pentamers and will notify the customer of any anticipated problem sequences.
Key Publication
Lepone L., et al. (2010). “Monofunctional and polyfunctional CD8+ T cell responses to human herpesvirus 8 lytic and latency proteins.”
Clin. Vaccine Immunol. 17(10): 1507-16. [PubMed ID 20719985]
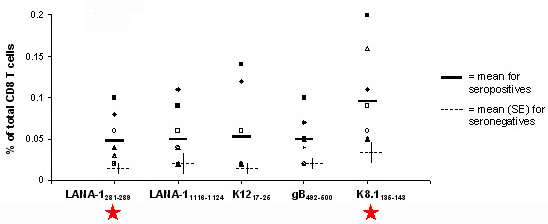
Figure 3. HLA A*02:01 ProVE® Pentamer staining in peripheral blood samples from 8 HHV-8-seropositive donors. PBMC from HLA A*02:01-positive HHV-8-seropositive donors were stained with ProVE® MHC Class I- Pentamer complexes specific for LANA-1281-289, LANA-11116-1124 K1217-25, gB492-500, and K8.1135-143. Newly-discovered epitopes are starred. Average response is indicated by the black bar and response levels in seronegative donors indicated by the dotted line. Read the full case study here.
Feature Comparison
ProVE® Pentamer Libraries |
Custom Pro5® Pentamers |
|
Target Applications |
Epitope screening Epitope validation, e.g. following ELISPOT analysis Mutagenesis studies |
Quantitative epitope confirmation Immune monitoring of confirmed epitopes |
Available Alleles |
HLA-A*01:01, 02:01, 03:01, 11:01, 24:02, 29:02 HLA-B*07:02, 08:01, 14:02, 15:01, 27:05, 35:01, 40:01 H-2 Kb, Db, Kd, Dd, Ld Mamu A*01, A*02 |
HLA-A*01:01, 02:01, 03:01, 11:01, 11:03, 24:02, 29:02, 68:01 HLA-B*07:02, 08:01, 14:02, 15:01, 27:05, 35:01, 35:08, 40:01, 51:01, 54:01 H-2 Db, Dd, Kb, Kd, Ld Chimeric A02:01/Kb Mamu A*01, A*02 |
Minimum Order |
10 ProVE® Pentamers | 1 Pro5® Pentamer |
Delivery Time |
2-3 weeks from receipt of peptides | Catalog items 1-2 weeks Custom items 4-6 weeks |
Custom Set Up Charge |
No | No |
Pack Size |
20-50 tests | 50, 150 or 500 tests |
Synthesis Process |
Rapid high throughput synthesis | Extended synthesis for maximum purity and quality |
Quality Control |
Full QC process incl. pass/fail QC provided for each reagent | Full QC process |
Fluorescent Labels |
Pro5® Biotag, R-PE, APC | Pro5® Biotag, R-PE, APC |
Labeling Method |
Supplied with separate Pro5® Biotag or Fluorotag (R-PE or APC), for two layer staining | Pre-conjugated with Biotin, R-PE or APC Or supplied with separate Pro5® Biotag or Fluorotag (R-PE or APC), for two layer staining |
Who Provides the Peptide? |
Provided by customer Or synthesized by ProImmune |
Synthesized by ProImmune for full quality control |
What Do I Receive? |
All ProVE® Pentamers that pass quality control | The custom Pentamer |
Guarantee Period |
3 months, stored at 4°C | Biotin, R-PE or APC labeled: 6 months, stored at 4°C Unlabeled: 12 months, stored at -80°C |
Comparative Staining of PBMC with ProVE® MHC Pentamers and Pro5® MHC Pentamers
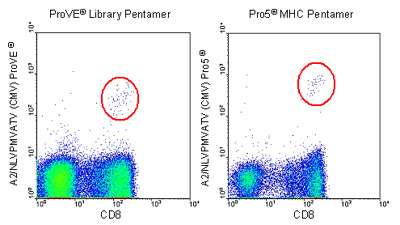
Peripheral blood lymphocytes were obtained from a healthy donor previously shown to respond to the A2-restricted CMV epitope, at approx. 0.15% of total PBMC. The left plot shows cells stained with the ProVE® MHC Pentamer, while the right plot shows cells stained with Pro5® MHC Pentamer made by the conventional process. The antigen-specific population is circled in red on both plots.
(Experimental procedure: cells were incubated with 1ug recombinant A2/CMV complex, followed by 1 test of PE-labeled Pro5® Fluorotag & 1 test of FITC-labeled anti-CD8 antibody (Clone LT8). Approximately 30,000 events are shown in each plot).
