Your basket is currently empty!
Advances-Toward
Advances Toward a Vaccine for Ebola Virus
ProImmune’s Antigen Characterization Platform Accelerates Development of a Vaccine for Ebola and Marburg Viruses
|
Gene Olinger, of the US Army Medical Research Institute of Infectious Diseases (USAMRIID), used the ProImmune Antigen Characterization and Biomarker Discovery Summit to present the very latest thinking on strategy for developing a vaccine for those exposed to filoviruses. There are several challenges to working with Marburg and Ebola viruses: their high infectivity and lethality have obviously impeded basic research, so relatively little is known of their virology, and further, they are highly species-specific. Researchers commonly rely on rodent and macaque models for the diseases, but for filoviruses, bats are strongly implicated as a reservoir species, while higher primates, ungulates and humans will die from the same strain. |
 Figure 1: Ebola virus transmission electron micrograph image colorized, taken at 160,000x magnification. |
There are five strains of Ebola virus, and Gene Olinger is working with the two most lethal, Zaire and Sudan. Ebola belongs to the filovirus family (figure 1); it is a single-strand RNA virus that produces 7 mRNAs upon infection of a host, including one encoding glycoprotein (GP). GP presents an attractive vaccine target as it is a surface protein, and likely to be involved in cell entry.
|
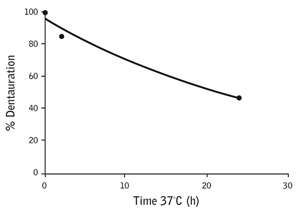
Figure 2: Denaturation curve for a complex of a single Ebola peptide with HLA-A*02:01, measured using the ProImmune REVEAL™ Rate Assay. |
In collaboration with Alphavax, scientists in the Olinger lab have been working with a Venezuelan equine encephalitis replicon (VRP) engineered to express filovirus GP. They believe this approach strikes a good balance between vaccine potency and safety. They want to evaluate the antigen components required to generate an immune response in humans. To identify potential T cell epitopes in Ebola Zaire GP, Dr. Olinger chose to use ProImmune’s REVEAL® & ProVE® Rapid Epitope Discovery System to analyze the MHC affinity and binding kinetics of a library of Ebola-derived peptides.
|
As a result of his project using the ProImmune REVEAL® platform, Dr. Olinger has a picture of the regions of Ebola Zaire GP that are likely to cause an immune response in HLA-A*02:01–positive human subjects. Three peptides in particular look to be potentially immunogenic, displaying the strong MHC binding and slow off-rates that, in ProImmune’s experience, are indicative of a good T cell epitope (figure 2).
This information will allow the team to understand the fine detail of an immune response to their vaccine candidate when it comes to be tested. Since ultimately they need to correlate an immune response with protection, this is useful information indeed. The Olinger lab’s next step will be a move into animal models with the newly identified epitopes. Ultimately they are working on developing immune assays to predict protection in vaccinated macaques that can be applied to human vaccine trials.
 |
Laura Pruger, who carried out much of the epitope validation work in the Olinger Lab, photographed in USAMRIID’s BSL4 testing facility in Frederick Maryland. This work was carried out at USAMRIID Read the paper here: Identification of novel HLA-A*0201-restricted CD8+ T-cell epitopes on Ebola Zaire glycoprotein (45.26) |
The information contained in this article does not necessarily reflect the position or the policy of the U.S. Government and no official endorsement should be inferred.