Your basket is currently empty!
Case-Study
Case Study:
ProImmune REVEAL® HLA-Peptide Binding Assay helps characterize peptides in Graves’ Disease
Muixi et al. (2008). Thyroglobulin Peptides Associate In Vivo to HLA-DR in Autoimmune Thyroid Glands.
J. Immunol. 181(1): 795-807. [PubMed ID 18566446]
To date there have been very few studies into HLA-DR epitopes that use human autoimmune tissue, mainly due to low availability of such tissues, low HLA-Class II expression and heterozygosity of samples. Despite these constraints, this study by Muixi et al. identified seven different HLA-DR-associated peptides belonging to thyroglobulin, a known target autoantigen in autoimmune thyroid diseases. The HLA-peptide binding assay helped to overcome the experimental limitations, as the assay is cell free and peptide sequences can be screened against a wide array of alleles. The assay was used to assess the binding of potential thyroglobulin epitopes to HLA alleles DRB1*15:01 and DRB1*03:01.
HLA Class II-peptide complexes were first purified from ex vivo thyroid samples of patients with Graves’ disease and the peptide sequences determined by mass spectrometry. Of the 150 peptides isolated with DR molecules, 7 thyroglobulin-specific peptides were identified. To verify the binding affinity to HLA- DRB1*15:01 and DRB1*03:01, the peptides were analyzed using the Class II HLA-peptide binding assay. The results showed that thyroglobulin (2098-2112) binds to DRB1*03:01 (see figure), which is in agreement with results from the ProPred binding analysis software. Also in support of these findings, other recent studies have demonstrated the pathogenicity of this epitope using a DR3 transgenic mouse model of experimental autoimmune thyroiditis.
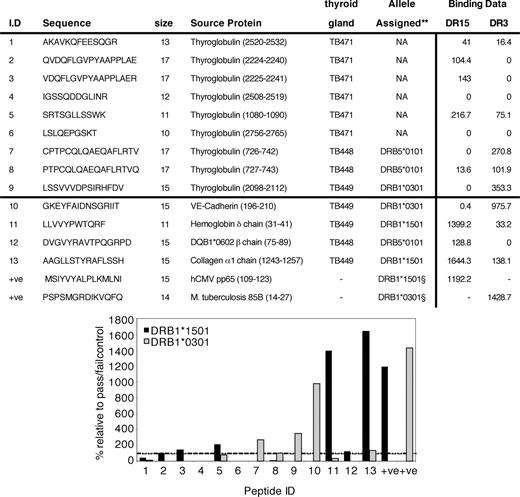
Figure: Results of the HLA-peptide binding assay. Thyroglobulin peptides (1-9) were tested alongside other peptides that had been theoretically assigned to one DR allele (10-13). Binding data are expressed as the percentage binding compared with a pass/fail peptide. Peptide 9 was confirmed as a medium-binder to DR3. The alleles assigned to peptides 10, 11 and 15 were also confirmed.
Copyright 2008 The American Association of Immunologists, Inc.
