Your basket is currently empty!
Decreasing-the-Hazards
Decreasing the Hazards of Bone Marrow Transplantation
Single-Antigen-Specific Donor Lymphocyte Infusion: A Promising New Way to Address the Risk of Opportunistic Infection Following Transplantation
 |
Professor Paul Moss of the CRUK Institute for Cancer Studies, Birmingham, UK, used his presentation at the ProImmune conference to give an overview of more than a decade’s work in his lab. Professor Moss was part of the team who laid the foundations for research into antigen-specific T cells through developing the very first MHC multimer. Today, his work benefits from the huge range of Pro5® MHC Class I Pentamers available from ProImmune. Looking to the future, he is particularly excited by the therapeutic potential of adoptive transfer of antigen-specific T cells. He presented stories from two very different immune scenarios. |
Much of the early work with MHC multimers was carried out in the cytomegalovirus (CMV) field, not least because CMV infection is very common and results in a large CD8+ T cell response and in some cases later in life more than 50% of the total CD8+ T cell repertoire. In some parts of the world, CMV infection is found in over 90% of the population, and is mostly latent, as the virus has evolved to co-exist in an immunocompetent host and under chronic immune control. Nonetheless, CMV infection has a dramatic effect on the CD8+ T cell repertoire. Throughout the lifetime of a ‘healthy’ CMV-infected individual, the presence of CMV causes an increase in the number of memory T cells and also accelerates the decline in number of naïve CD8+ T cells seen with age. The exact mechanisms of this switch are unclear, although they may well contribute to a clinically observable effect – evidence is emerging that elderly CMV carriers have a reduced life expectancy. Professor Moss and his team are characterizing CMV-specific CD8+ T cells using multimer staining in conjunction with cytokine analysis to begin to understand how CMV infection modulates the T cell repertoire.
More severe problems arise when the host becomes immunocompromised. Opportunistic infections in stem cell transplantation still account for a mortality rate of up to 15% in stem cell transplant patients over five years following the transplant. In the early days of stem cell transplantation, CMV was a major cause of patient death, as patients have very few CMV-specific T cells following myeloablative treatment. Adoptive transfer of donor CMV-specific T cells to correct this becomes a very attractive option; the cells will not be rejected as the recipient remains largely immunocompromised, and the cells can expand and clear CMV viremia in their new host. Professor Moss demonstrated that MHC tetramers in conjunction with magnetic beads can be used to purify epitope-specific T cells for this style of treatment and cited a study where a patient who had been suffering from CMV for a few weeks, was resistant to drugs and had no T cells in their blood, was given CMV epitope-specific T cells. Nine days later T cells were present and the patient became CMV-negative (1).
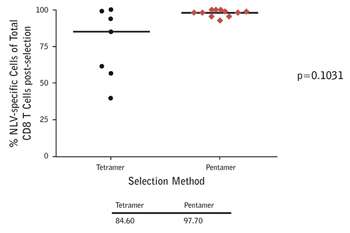 |
Figure 1: Anti-biotin magnetic bead-based separation of CMV epitope-specific T cells from donors using A*02:01/NLVPMVATV multimers. The purity of selection is much greater with ProImmune Pentamers versus tetramers. As a consequence cells prepared using Pentamers will be much more effective when used in adoptive transfer than cells separated using tetramers, which will contain many more non-specific T cells. The viability of the selected cells was also shown to be excellent. |
Professor Moss has found that he obtains the cleanest separation of cells using ProImmune Pentamers, compared with tetramers for the same epitopes (Figure 1).
Similar work with tumour antigen-specific CD8+ T cells has raised more questions than answers. Cancer Testis Antigens (CTAgs) are expressed solely by germline cells. The existence of a testis-blood barrier means that the testes remain immune-privileged and tolerance is not established against CTAgs. Some tumours acquire expression of CTAgs, and an immune response is sometimes mounted against them. However, CTAg-specific CD8+ T cells are much more difficult to find than their CMV-specific counterparts. In a recent study, 15 out of 37 myeloma patients showed anti CTAg-specific T cell response which measured between just 0.01 and 0.7% of the CD8+ T cell pool (2). If these cells could be expanded ex vivo, could they be adoptively transferred with therapeutic benefit? In a case reported several years ago, two patients receiving kidneys from the same donor both developed melanomas in those kidneys. Investigation found that the donor had had a small melanoma removed 16 years earlier (3). This suggests that the tumors were transferred with the transplant, but that a population of CD8+ T cells that remained in the donor actively suppressed the expansion of sub-clinical tumors. It is a possibility that the CD8+ T cells were targeting CTAgs. Presumably then, receipt of tumor-specific CD8+ T cells from the same donor could send a tumor into remission. Professor Moss questions this idea because his work has shown that the CD8 response actually increases during terminal stages of cancer. He believes that more work is needed to correlate immune response with clinical outcome before adoptive transfer is applied to treat cancer and MHC multimers, such as Pro5® MHC Pentamers, will play a key role in investigating these questions further.
Paul Moss works at the Division of Cancer Science, University of Birmingham.
Watch a presentation by Prof. Moss at a ProImmune conference
(1) Cobbold, M., et al., (2005) Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA–peptide tetramers. J. Exp Med 202: 379-386 [PubMed ID 16061727]
(2) Goodyear, O., et al., (2008) Differential pattern of CD4+ and CD8+ T-cell immunity to MAGE-A1/A2/A3 in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. Blood 112: 3362-72 [PubMed ID 18658027]
(3) MacKie, R., et al., (2003). Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. New England Journal of Medicine 348: 567-568 [PubMed ID 12571271]
