Your basket is currently empty!
Pentamers-integral
Case Study:
Pro5® Pentamers integral in successful research into novel immunization strategy
Yagi, H. et al. (2006). Induction of Therapeutically Relevant Cytotoxic T Lymphocytes in Humans by Percutaneous Peptide Immunization. Cancer Research. 66(20): 10136-10144. [PubMedID: 17047078]
In this study by Yagi et al. a novel percutaneous immunization strategy was used to deliver a peptide vaccine to melanoma patients. This method of percutaneous peptide immunization (PPI) used epidermal Langerhans cells as antigen presenting cells to deliver the vaccine effectively to the immune system. The outer layer of the epidermis was removed in order to increase the permeability of the skin to the peptide vaccine and allowed the maturation of the Langerhans cells for enhanced antigen presentation.
Pro5® MHC Pentamers for MAGE-2 (A*24:02 / EYLQLVFGI), and tyrosinase (A*24:02 / AFLPWHRLF), and a custom Pro5® MHC Pentamer for MAGE-3 (A*24:02 / IMPKAGLLI), were used to monitor frequencies of antigen-specific T cells in patients after immunization. After several rounds of immunization, staining with Pro5® MHC Pentamers indicated that antigen specific responses increased over the course of 6 months in both melanoma patients and healthy controls.
Figure 1
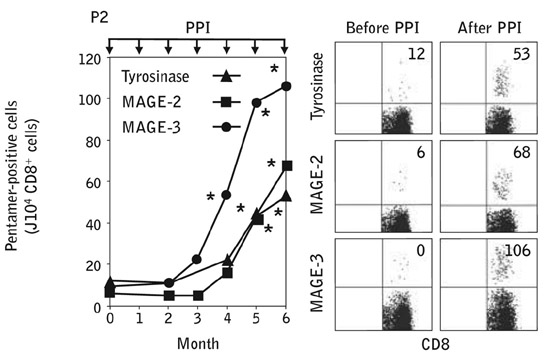
Figure 1: Induction of Pentamer-positive CD8+ T cells. PBMCs obtained 7 days after each PPI were immunophenotyped with Pentamers for tyrosinase, MAGE-2, and MAGE-3 and analyzed by flow cytometry.
Further analysis of tumor infiltrating leukocytes in one of the melanoma patients showed that the MAGE-2, MAGE-3 and tyrosinase specific CD8+ cells were present in these samples.
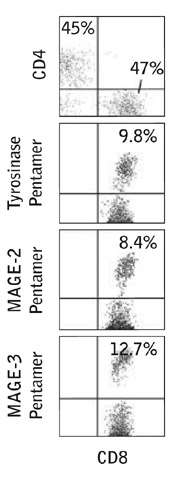 |
Figure 2: In vivo infiltration of CTLs into melanoma lesions induced by PPI. Flow cytometric profiles of cell suspensions obtained from a regressing s.c. nodule in patient 2 during PPI, showing the percentage of CD4+ and CD8+ cells among CD45+ cell-gated leukocytes and the percentage of Pentamer+ cells among the CD8+ CD45+ cell-gated populations.
The presence of these Pentamer-positive cytotoxic T cells coincided with a decrease in tumor size and progression indicating that the percutaneous peptide vaccine delivered to barrier-disrupted skin was effective, as well as being safe and non-invasive.
|
