Your basket is currently empty!
ProImmune-Pro5-MHC
Case Study: ProImmune’s Pro5® MHC Pentamers are used to demonstrate that allogeneic T cells may be targeted to B cell leukemia
 |
Abrahamsen, et al. (2010). Targeting B cell leukemia with highly specific allogeneic T cells with a public recognition motif. Leukemia 2010 Nov;24(11):1901-9. [PubMed ID 20844564]
Olweus lab members: Sébastien Wälchli, Ingerid W. Abrahamsen and Johanna Olweus |
The intelligent design of immunotherapy for B cell leukemia represents a significant challenge. Any potentially cancer–reactive T cells will have been deleted from the repertoire of the patient during maturation of their immune system, as the B cell antigens borne by the leukemia cells are by their nature self antigens.
However, T cell receptors are inherently reactive towards MHC. Building on this, Abrahamsen et al. investigated whether foreign HLA molecules bearing self B cell peptide antigens could provoke a response from patient-derived T cells against their own B cell antigens, and so potentially eliminate cancerous B cells. It has been suggested that such alloreactive T cells will respond to a wide repertoire of peptides, so the team first sought to address the important question of peptide specificity: would these T cells respond to any peptide in the context of foreign MHC, or be sufficiently restricted to a single B cell antigen to be of potential therapeutic value?
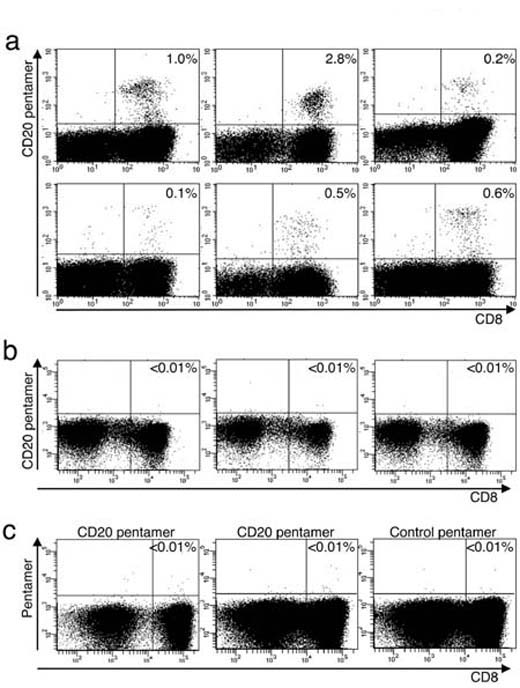
T cells reactive with a peptide derived from the B cell antigen CD20 (SLFLGILSV) in the context of HLA-A*02:01 could be readily obtained from HLA-A*02:01-negative donors. Abrahamsen et al. used their established method (Stronen et al., Scand J Immunol. 2009, 69:319-28) of co-culturing T cells from A*02:01-negative donors with A*02:01-positive CD20 peptide –pulsed antigen presenting cells to successfully generate A*02:01/CD20-specific T cell lines. A custom ProImmune Pro5® A*02:01/ SLFLGILSV MHC class I Pentamer was used to identify the antigen-specific T cell population.
|
|
|
Figure 1: Representative flow cytometry analysis plots to show (a) A*02:01/SLFLGILSV Pro5® Pentamer-reactive cells from A*02:01-negative donors on day 19 after the start of coculture with A*02:01-transfected, SLFLGILSV peptide-pulsed dendritic cells. (b) A*02:01/SLFLGILSV Pentamer staining of freshly-isolated PBMC from the same donor and (c) A*02:01-positive donor PBMC subject to the same treatment as those in (a). |
The same method used with A*02:01-positive donors failed to expand a similar population of antigen-specific T cells (Figure 1c), confirming that A*02:01-positive individuals are tolerant of this self antigen.
To demonstrate the specificity of their antigen-reactive T cells, Abrahamsen et al. used a library of 42 A*02:01 Pentamers, the majority of which were ProImmune ProVE® Pentamers, each displaying an irrelevant peptide with homology to SLFLGILSV. The two cell lines tested stained positively with the A*02:01/SLFLGILSV Pentamer, but negatively for the irrelevant peptides, confirming their antigen specificity. In order to assess the fine specificity of their T cell lines, the team took a scanning alanine substitution approach, using a panel of A*02:01 multimers. The motif of four central amino acids from the SLFLGILSV epitope were found to be most critical for binding, since binding of the cells to Pentamer was most reduced when these residues were substituted for alanine. Different isolated T cell line clones showed variable dependency on other residues, but all relied strongly on the core four amino acids for multimer binding.
As the most stringent test of specificity, Abrahamsen et al. next measured the reactivity of their T cell lines towards cells co-expressing A*02:01 and CD20. The team used various experimental scenarios to show that their cells were indeed selectively antigen reactive. As an example, the T cell lines were reactive to HEK 293 cells only when the HEK 293 cells were cotransfected with A*02:01 and CD20, and crucially not with A*02:01 alone or with A*02:01 and irrelevant control antigen. This evidence indicated that the CTL lines were recognizing naturally processed and presented CD20 antigen.
In a final proof of principle, the CD20-reactive T cells were tested for their ability to kill cells from the peripheral blood of chronic lymphoid leukemia (CLL) patients. Encouragingly, robust levels of specific lysis were observed with A*02:01-positive target cells, but A*02:01-negative CLL cells were spared.
The high functional avidity of the CTLs isolated and characterized by Abrahamsen et al. using ProImmune MHC Pentamer technology is truly encouraging. The use of alloreactive T cells to induce a target-specific graft-versus-leukemia response without simultaneously inducing graft-versus-host disease marks a promising start for the future of cancer immunotherapy.
This work was carried out in the Olweus Lab, Oslo University Hospital: