Your basket is currently empty!
Rate assays
Case Study:
ProImmune REVEAL® Rate assays and Pro5® Pentamers central to study investigating oxidative stress
Weiskopf, D. et al. (2010). Oxidative Stress can alter the antigenicity of immunodominant peptides.
J. Leukocyte Biol. 87: 165-172. [PubMed ID: 19801502]
Oxidative stress mediated by reactive oxygen species such as hydrogen peroxide can be a factor in age-related diseases such as Alzheimer’s and Parkinson’s disease. To model the effects of oxidative stress upon proteins, Weiskopf et al. investigated how an immunodominant Cytomegalovirus (CMV) epitope (A*02:01/NLVPMVATV) was affected by oxidation of the methionine residue. CMV was selected as a target because latent CMV infection affects 60-100% of the adult population.
NLVPMVATV peptide was incubated with hydrogen peroxide to specifically oxidize the sulfur containing methionine residue (NLVPM(-ox)VATV). To determine whether this modification affects the peptide binding to the A*02:01 MHC complex, ProImmune REVEAL® Complete Rate Assays were carried out on both the un-modified and oxidized peptides. The on- and off-rates of the peptide were very similar indicating that methionine oxidation does not impair binding to the A*02:01 MHC complex. These results were further validated by a T2 binding assay.
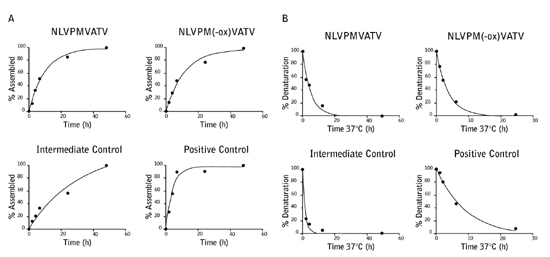
Figure 1: The on-rate (A) and off-rate (B) of the NLVPMVATV and NLVPM(-ox)VATV peptides to the A*02:01 MHC complex were measured over 48 hours (on-rate) and 24 hours (off-rate) and compared to 2 known T cell epitopes with strong and intermediate binding.
The binding avidity of the antigen specific cells for the two peptides was assessed using a custom synthesized Pro5® Pentamer (A*02:01/NLVPM[-ox]VATV) and a catalog Pro5® Pentamer (A*02:01/NLVPMVATV). Dissociation of the Pentamer complex from the antigen-specific T cells was measured up to 3 hours after incubation and it was found that the NLVPM(-ox)VATV Pentamer complex had a significantly lower avidity than the native sequence.
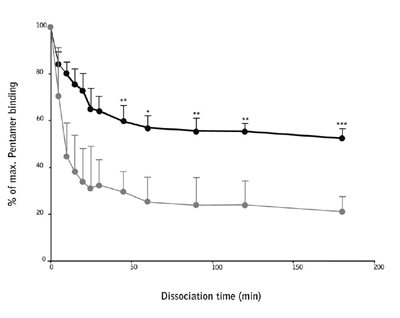
Figure 2: CD8+ T cells were stimulated with NLVPMVATV (black line) or NLVPM(-ox)VATV (gray line) peptide for 2 weeks and then stained with their respective Pentamer. Dissociation rates of the Pentamers were assessed at time points up to 3 hours.
In addition to the lower binding avidity, NLVPM(-ox)VATV peptide also induced a much lower level of proliferation after 7 days of culture than the un-modified peptide. This finding was confirmed using Pro5® Pentamer staining.
It was demonstrated that while small oxidative changes to the peptide sequence may not affect the binding of the peptide to the MHC complex, there might be adverse consequences on downstream responses such as reduced T cell proliferation and functionality. This could lead to the development of viral persistence, whereby virus-infected cells are not eliminated in diseases such as CMV or EBV. For this study, ProImmune REVEAL® Rate Assays and Pro5® Pentamers were pivotal in determining the effects of oxidation upon the binding, avidity and proliferative capacity of a well-characterized CMV immunodominant epitope.
