Your basket is currently empty!
The Human Side
The Human Side of Counter-Bioterrorism
T Cell Proliferation Assays Validate Humanization of an anti-VEEV Antibody
O’Brien, LM. et al. (2012). A humanised murine monoclonal antibody protects mice from Venezuelan equine encephalitis virus, Everglades virus and Mucambo virus when administered up to 48h after airborne challenge. Virology. 426(2): 100-5. [PMID:22341308]
Venezuelan Equine encephalitis virus (VEEV) was previously developed as a biological weapon by several nations and it is currently assessed to be an agent with potential for bioterrorist use. In nature, VEEV is a mosquito-borne pathogen that affects equine species but human outbreaks have occurred in Mexico, Colombia, Venezuela and the United States. There is concern that the deliberate release of VEEV would cause human casualties, particularly since no medical countermeasures (vaccines or antivirals) exist.
To address this potential threat, UK Government scientists at Dstl (Defence Science and Technology Laboratory) began work on a virus-neutralising, protective antibody. They used a mouse antibody as the basis for an antibody that could be employed for human treatment.
Introducing a mouse antibody into humans is fraught with difficulty. The human immune system recognizes murine antibodies as foreign and so mounts a response against them. Accordingly, the Dstl Team took one of the most broadly-reactive murine monoclonal antibodies and modified it to remove as much ‘mouse’ sequence as possible. They created two versions, a chimeric mouse-human protein and a fully humanized version created through Complementarity Determining Region (CDR) grafting using human germline IgG framework regions.
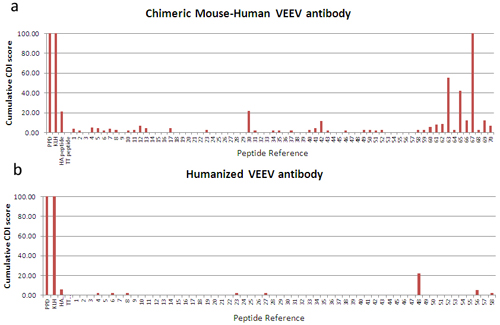
Figure 1: CD4 T cell proliferative responses to control antigens (PPD, KLH, TT and HA), and peptide libraries derived from the sequences of Dstl’s two antibodies (chimeric, top, and humanized, bottom). The cell division index (CDI) is determined as a ratio of the proportion of CFSE-dim [divided] cells in the stimulated versus unstimulated samples. 40 different donor samples were tested and the cumulative results are shown. Lower scores are indicative of reduced potential antigenicity in humans. Data reproduced by kind permission of Dstl.
Before considering whether to move the treatment into clinical trials, the Dstl team wanted to gather as much information as possible on the potential immunogenicity of their novel antibody construct. For this, they used ProImmune’s Immunogenicity services and requested that ProImmune test both antibodies in CFSE T cell proliferation assays. Briefly, CD4+ T cell proliferation in response to overlapping peptide libraries generated from the sequences of each antibody was assessed in a panel of 40 donors. At the outset, cells are labeled with membrane dye CFSE and this is diluted as the cells divide. The graph in figure 1 shows a summary of responses to each antibody. The results presented show the cumulative antigenicity measured for all study donors. Potential T cell epitopes present in the chimeric antibody have clearly been removed through the humanization process, a demonstrable success. Not all humanization projects are so successful so it remains important to test the efficacy of the process each time through prospective immunogenicity testing.
In work published in the journal ‘Virology’ the scientists at Dstl showed that the humanized antibody is an effective treatment in VEEV-exposed mice. Even when the antibody was administered 48 hours after viral challenge, antibody treatment was sufficient to allow the test cohort of mice to survive, whilst VEEV-exposed untreated mice succumbed to the virus. If the treatment is to progress, the next step will be to move the humanized antibody to non-human primate models before progressing to human clinical trials. It is possible that this new therapy has the potential to allow a fight back against the consequences of infection with this virus.
